Introduction: Both aggressive T and B cell lymphoma may harbour Anaplastic Lymphoma Kinase (ALK) translocations: ALK+ Anaplastic Large Cell lymphoma (ALCL) offer a good outcome with first line therapy with 5-year survival of 79%, but relapses occur in half of the patients (pts) and salvage therapy still represents a challenge for clinicians; ALK+ large B cell lymphoma (LBCL) are very rare cases but with dismal prognosis. In addition other hematologic neoplasms can be caused by ALK fusions.
Crizotinib, an ALK/MET tyrosine kinase inhibitor (TKI), provides long-term response in up to 70% relapsed/refractory (R/R) ALK+ ALCL and can obtain a similar but less durable response rates in ALK+ LBCL. In both cases the loss of response to crizotinib is frequently a fatal event. Lorlatinib, a selective ALK and ROS1 TKI, demonstrated preclinical inhibition of all reported ALK kinase domain mutations responsible for resistance to Crizotinib.
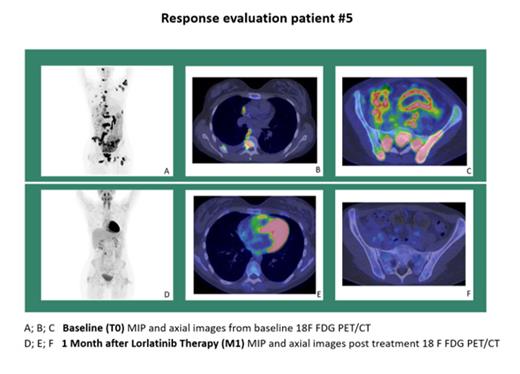
Methods: From 2019 to 2022 six relapsed/refractory ALK + neoplasm pts previously treated with at least one ALK-inhibitor were enrolled in a phase 2 open label monocentric study with lorlatinib monotherapy (EudraCT2016-003970-41): Three patients had diagnosis of ALK+ LBCL (# 1, 2, 3) and 2 (#5 and #6) had ALK+ ALCL. One patient (#4) with an ALK+ histiocytosis was also included. Median age was 22 years (range 19-57), Ratio M/F was 1, all pts were in stage IV. Median prior therapy lines was 3 (range 2-5); all pts previously received Crizotinib, two pts received also Alectinib and 1 Ceritinib as TKI treatment. Lorlatinib was administered daily at dose of 100 mg. Clinical, laboratory, radiological and molecular data were collected at screening and during scheduled follow-up. Response was evaluated with 18F-FDG PET/CT (and with contrast enhanced (ce) CT scan) according Lugano Classification, and with ALK RT-PCR on peripheral blood and bone marrow. Side effects were classified according to CTCAEv4.03. PFS and OS were determined from Lorlatinib start to relapse and/or death. The primary endpoint was the evaluation of ORR considering 18F-FDG PET/CT scan and ALK- RT-PCR. Secondary endpoints were: PFS, OS, toxicity, QoL.
Results: ALK rearrangement fusion pattern was detected in 6/6 pts: 3 pts with LBCL harboured Clathrin-ALK (CTCL::ALK) rearrangement and 2 ALCL pts the ATIC::ALK fusion; the ALK+ histiocytosis patient harbored an EML4::ALK rearrangement. Median follow-up was 13.7 months (IQR 22-55). At month +1 (M1) 3/6 patients had complete response (CR) defined as complete metabolic and complete radiologic response (CMR/CR) according to PET/CT scans and ALK RT-PCR negativity. The remaining 3 pts had a partial response (PR), for an Overall Response Rate (ORR) of 100% (95%CI: 0.6-1.0). One pt is still on treatment and in CR at 45 months, receiving Lorlatinib 100 mg daily with neither toxicities nor need for Allogenic Stem Cell Transplantation (ASCT); 2 CR pts underwent ASCT after 1 month, resumed lorlatinib after ASCT and are still in CR. 2 pts with PR relapsed within month +3: one pt received salvage therapy without benefit, the other one underwent ASCT but progressed 3 months later. Both pts had ALK+ BCL with CTCL-ALK rearrangement. 1 pts in PR AT M1 (ALK+ istiocytosis) obtain CR at M3 after surgical resection of a residual lung mass and is still receiving Lorlatinib at month 36. Median PFS and OS were not reached: PFS at 6 and 12 months is 83% and 62% respectively; OS at 6 and 12 months is 83% and 55%. Safety: 2/6 pts had thrombocytopenia grade 1, whereas 3/6 pts showed grade I-II hypertriglyceridemia/hypercholesterolemia and muscle cramps.
Conclusions: Lorlatinib represents a safe and effective salvage therapy for ALK+ Lymphoma patients failing first line TKI treatment. The obtainment of CR at M1 seems to represent a pivotal prognostic factor. Lorlatinib also offers a promising bridging regimen to salvage ASCT especially for ALK+ BCL patients, with high response rates and no additional toxicities, .
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Lorlatinib, a selective TKI of ALK and ROS1, demonstrated preclinical inhibition of all reported ALK kinase domain mutations responsible for resistance to Crizotinib in ALK + neoplasms.